About Us
X-Chromosome Inactivation (XCI) is one of the best-characterized epigenetic phenomena where a transcriptional memory of chromosome silencing persists with each cell division, from the early embryo into adulthood. The silencing of one X chromosome involves many epigenetic players, including long noncoding RNAs, DNA methylation, exchange of histone tail modifications, and histone variants.
Female mammals (XX) have two X-chromosomes, and one X is randomly chosen for transcriptional silencing in order to equalize the expression of X-linked genes compared to males (XY). Thus females are mosaic for X-chromosome expression, where a cell expresses either the maternal or paternal X. One visual manifestation of female X-mosaicism are calico cats, as coat color genes are X-linked.
Sex Differences with Immune Responses. There are significant sex-based differences in immune responses to pathogens and self-antigens. Women exhibit increased susceptibility to various autoimmune diseases, and men have preferential susceptibility to some viral, bacterial, parasitic, and fungal infections. While sex hormones clearly contribute to sex differences in immune cell composition and function, the presence of two X chromosomes in females suggests that differential gene expression of numerous X-linked immune-related genes may also influence sex-biased innate and adaptive immune cell function in health and disease.
Calico cats, usually female, visually demonstrate X-mosaicism (either mom’s or dad’s X is expressed) that arises from X-Chromosome Inactivation. The genes for coat color in cats are X-linked.
Mouse fibroblasts have Xist RNA ‘clouds’ that co-localize with heterochromatic modifications such as H3K27me3.
XCI & Gene Escape. XCI is initiated during early embryonic development, during which one X chromosome is randomly selected for transcriptional silencing, generating an inactive X chromosome. In most somatic cells, transcriptional silencing of the Xi is maintained with each cell division through Xi enrichment of Xist RNA, heterochromatic histone marks, and DNA methylation, some of which can be visualized cytologically using RNA FISH and immunofluorescence.
Although most genes on the Xi are silenced, about 15-23% of X-linked genes in humans and ~3-7% of X-linked genes in mice are transcribed from the Xi and ‘escape’ XCI, either constitutively or in a cell type-specific manner. XCI gene escape varies considerably by cell type, as well as between individual cells, and among individuals. Additionally, some XCI escape genes display sex-biased expression.
Dynamic XCI maintenance. We recently discovered that XCI is maintained differently in female lymphocytes in a process we term ‘dynamic XCI maintenance’. Naïve T and B cells, which are quiescent, lack cytological enrichment of Xist RNA and heterochromatin marks on the inactive X. After lymphocyte stimulation in vitro, these epigenetic modifications re-localize back to the inactive X. Xist RNA is continuously transcribed yet the RNA transcript localization varies by cellular state. This dynamic localization of Xist RNA and heterochromatic marks to the Xi is also observed during lymphocyte development, as Xist RNA is lost from the Xi after the common lymphoid progenitor stage, at the pro-B cell and DN1 thymocyte stages, and Xist transcription remains constant.
Non-canonical XCI maintenance is a feature of both adaptive and innate immune cells. Xist RNA exhibits substantial diversity in the robustness of its relocalization to the Xi depending on the type of immune cell.
Research Areas
Epigenetic Mechanisms of XCI maintenance in Immune Cells
We investigate the epigenetic mechanisms that regulate allele-specific gene expression from each X chromosome. Using single-cell microscopy, we discovered that epigenetic modifications are dynamically recruited to the inactive X chromosome following activation of T and B cells. We are investigating how gene expression is regulated across the inactive X chromosome following antigen stimulation and the epigenetic modifications and chromosomal structural changes that take place.
Female Biased Autoimmune Diseases and XCI maintenance
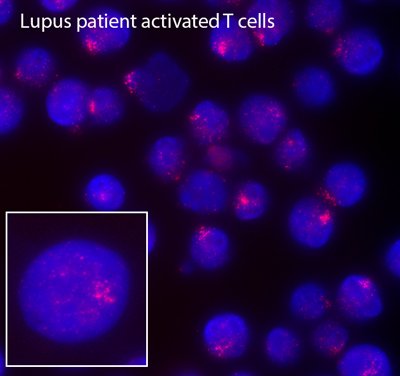
We discovered that Xist RNA localization and enrichment of heterochromatic modifications on the inactive X is perturbed in lymphocytes from female lupus patients and various mouse models of spontaneous lupus-like disease. These alterations are correlated with abnormal expression of X-linked genes which should be silenced and also genes that should escape XCI silencing. We are investigating the epigenetic features of the inactive X in other autoimmune diseases exhibiting a female bias where immune cells have altered expression of X-linked genes.
We have proposed the hypothesis that aberrant XCI maintenance mechanisms in lymphocytes result in partial reactivation of immunity-related genes on the inactive X. We are testing this hypothesis using mouse models of spontaneous autoimmune disease, models of induced autoimmune disease, and human patient samples.
Sex Differences with Immune Responses
Sex differences in the incidence or severity of infectious diseases have also been observed across a variety of distinct pathogens. Biological sex impacts pathogen replication and transmission, as well as the host immune response to the pathogen. The sex bias of infectious disease is typically explained by the stronger female immune response, which results in higher levels of inflammation. Mouse and rodent models often recapitulate the observed sex bias in humans and can be used to identify sex-specific molecular pathways resulting from infection of some pathogens. Pathogen type also influences infection incidence and disease severity, often exhibiting a sex bias in both humans and rodent models. Males typically exhibit greater incidence for most bacterial, parasitic, some viral, and fungal infections; females have greater incidence of parasitic worm infections.
Accumulating evidence suggests that XCI escape of some X-linked immunity genes in innate and adaptive immune cells contributes towards increased female protection from bacteria and parasites. The importance of X-linked immunity related genes for sex-biased responses to pathogens is underscored by observations of sex-specific phenotypes in genetic deletion models or in patients with specific mutations following infections.
Research Techniques
Microscopy: Nuclear imaging of Xist RNA and the X-chromosomes using RNA and DNA in situ hybridization (FISH); immunofluorescence detection of heterochromatic histone tail modifications
Allele-specific transcriptional, epigenomic, and spatial profiling of the inactive X and active X chromosome across somatic cell types (RNA-seq, CUT&RUN, ChIP-seq, Hi-C, DNA methylation)
Mouse models of autoimmune disease, where animals spontaneously develop lupus-like disease symptoms; Chemical induction of autoimmune disease phenotypes.
Human immune cell spectral flow cytometry profiling of patients with female-biased autoimmune diseases; epigenomic & transcriptional profiling of the X chromosomes from patient samples.